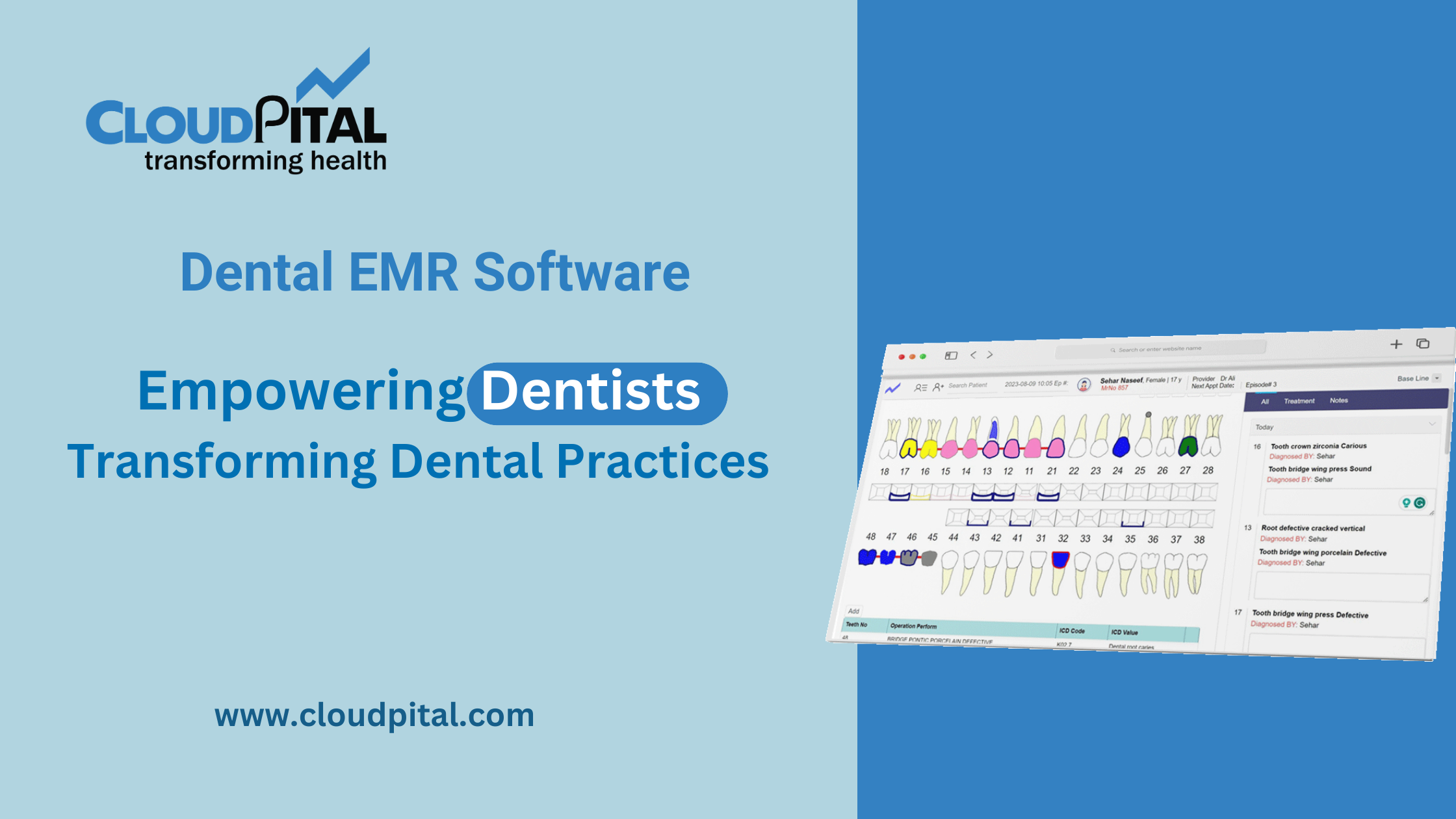
Cloudpital # 1 is one of the top Medical Solutions in Saudi Arabia are subject to stringent regulatory requirements to ensure patient safety, data security, and compliance with national healthcare standards. These regulations encompass various aspects of medical devices, software applications, and healthcare services. Understanding and adhering to these regulatory requirements is essential for companies developing, marketing, and implementing medical solutions in the Kingdom. Below are the key regulatory requirements for medical solutions in Saudi Arabia:
Click to Start Whatsapp Chatbot with Sales
Mobile: +966547315697
Email: sales@cloudpital.com
Cloudpital # 1 Medical Solutions in Saudi Arabia
Saudi Food and Drug Authority
The SFDA is the regulatory body responsible for overseeing the registration, marketing, and surveillance of medical devices, pharmaceuticals, and Medical Solutions in Saudi Arabia products in Saudi Arabia. Medical solutions, including software applications intended for medical use, must obtain regulatory approval from the SFDA before being marketed or distributed in the Kingdom. The SFDA ensures that medical solutions meet safety, efficacy, and quality standards through a rigorous review process.
Medical Device Regulations
Medical devices, including software applications used for medical purposes, are classified based on their intended use, risk level, and mode of action. The SFDA classifies medical devices into different risk classes (I, IIa, IIb, III) and imposes specific regulatory requirements for each class. Medical software applications, such as diagnostic tools, monitoring systems, and treatment planning software, fall under the purview of medical device regulations and must comply with applicable standards and guidelines.
Quality Management Systems
Companies developing and manufacturing medical solutions in Saudi Arabia are required to implement quality management systems (QMS) to ensure the consistent production of safe and effective products. International standards such as ISO 13485:2016 outline the requirements for QMS in the medical device industry, including design control, risk management, document control, and post-market surveillance. Compliance with QMS standards is essential for obtaining regulatory approval and maintaining product quality throughout the product lifecycle.
Data Security and Privacy
Medical Solutions in Saudi Arabia that handle sensitive patient data must comply with data security and privacy regulations to protect patient confidentiality and prevent unauthorized access or disclosure of health information. In Saudi Arabia, healthcare providers and technology vendors must adhere to the Health Insurance Portability and Accountability Act (HIPAA) standards, as well as local data protection laws and regulations. This includes implementing robust encryption, access controls, and data breach response protocols to safeguard patient information.

Interoperability Standards
Interoperability is critical for seamless data exchange and communication between different medical systems, devices, and applications. PMS in Saudi Arabia are expected to adhere to interoperability standards and protocols to ensure compatibility with existing healthcare infrastructure and facilitate information sharing across healthcare settings. Common interoperability standards include Health Level Seven (HL7), Integrating the Healthcare Enterprise (IHE), and Fast Healthcare Interoperability Resources (FHIR).
Clinical Validation and Evidence
Medical solutions must undergo rigorous clinical validation and evidence generation to demonstrate their safety, efficacy, and clinical utility. Clinical validation involves conducting clinical studies, trials, or real-world evaluations to assess the performance and impact of the medical solution on patient outcomes. The SFDA may require companies to submit clinical data and evidence supporting the effectiveness of their medical solutions as part of the regulatory approval process.
Post-Market Surveillance
Once medical solutions are commercialized and deployed in healthcare settings, ongoing post-market surveillance is essential to monitor their performance, identify adverse events or safety concerns, and implement corrective actions as needed. The SFDA requires manufacturers and healthcare providers to report adverse events, malfunctions, or incidents related to medical solutions and take appropriate measures to mitigate risks and ensure patient safety.
Ethical and Legal Considerations
EHR Systems in Saudi Arabia must comply with ethical and legal standards governing healthcare practices, research, and patient care. This includes obtaining informed consent from patients participating in clinical trials or using medical solutions, respecting patient autonomy and privacy rights, and adhering to professional codes of conduct and ethical guidelines. Healthcare providers and technology vendors must uphold ethical principles and act in the best interests of patients at all times.
Conclusion
Navigating the regulatory landscape for medical solutions in Saudi Arabia requires a comprehensive understanding of the SFDA regulations, international standards, and ethical principles governing healthcare practices. By complying with regulatory requirements, companies can ensure the safety, effectiveness, and quality of their medical solutions while contributing to improved patient care and outcomes in the Kingdom.
Click to Start Whatsapp Chatbot with Sales
Mobile: +966547315697
Email: sales@cloudpital.com
Medical Solutions in Saudi Arabia
Medical Solutions in Saudi Arabia
Medical Solutions in Saudi Arabia
Regulatory requirements for Medical Solutions in Saudi Arabia? similar software solutions prices were updated on 2025-06-15T19:33:28+00:00 in Saudi Arabia in Mecca, Medina, Riyadh, Khamis Mushait, Yanbu, Jeddah, Dammam, Unaizah, Uqair, Ha’il, Ta if, Al Bahah, Dhahran, King Abdullah Economic City, Najran, Diriyah, Qatif, Khafji, Jubail, Abqaiq, List of Cities and Towns in Saudi Arabia, Ras Tanura, Turubah, Jazan Economic City, Knowledge Economic City, Medina, Khobar, Abha, Tabuk, Saudi Arabia, similar software solutions prices were updated on 2025-06-15T19:33:28+00:00 We also provide in Saudi Arabia services solutions company in Hafar Al-Batin, Udhailiyah, Al-Awamiyah, Hofuf, Hautat Sudair, Buraidah, Tayma, Duba, ‘uyayna, Saihat, Al-Kharj, Al-ula, Jizan, Rumailah, Ar Rass, Arar, Shaybah, Al Majma’ah, Rabigh, Dhurma, Haradh, List of Saudi Cities by Gdp Per Capita, Badr, Sudair Industrial City, Baljurashi, Shaqraa, Al-Khutt, Habala, Ad Dawadimi, Dawadmi, Layla, similar software solutions prices were updated on 2025-06-15T19:33:28+00:00 Price is SAR 100 and this was updated on updated on 2025-06-15T19:33:28+00:00 similar Regulatory requirements for Medical Solutions in Saudi Arabia? software solutions prices were updated on 2025-06-15T19:33:28+00:00 in Saudi Arabia in Haql, Afif, Al-Abwa, Farasan, Al-Jaroudiya, Thadig, Al-Thuqbah, Al Wajh, Almardmah, Al-Zilfi, Muzahmiyya, Prince Abdul Aziz Bin Mousaed Economic City, Tharmada’a, Skaka, Um Al-Sahek, Sharurah, Tanomah, Bisha, Dahaban, Al Qunfudhah, Qurayyat, Saudi Arabia, Ha’ir, as Sulayyil, Al Lith, Turaif, Al-Gway’iyyah, Samtah, Wadi Ad-Dawasir, Az Zaimah, Safwa City, Jalajil, Harmah, Mastoorah, Hotat Bani Tamim, Jabal Umm Al Ru’us, Rafha, Qaisumah, Al-Ghat, Hajrah, Al-Hareeq. Excerpt: Jeddah (also spelled Jiddah, Jidda, or Jedda; Arabic: Jidda) is a Saudi Arabian city located on the coast of the Red Sea and is the major urban center of western Saudi Arabia similar software solutions prices were updated on 2025-06-15T19:33:28+00:00 Price is SAR 100 and this was updated on updated on 2025-06-15T19:33:28+00:00
29-5-2024